Chemical Production
A reactor with a good energy balance
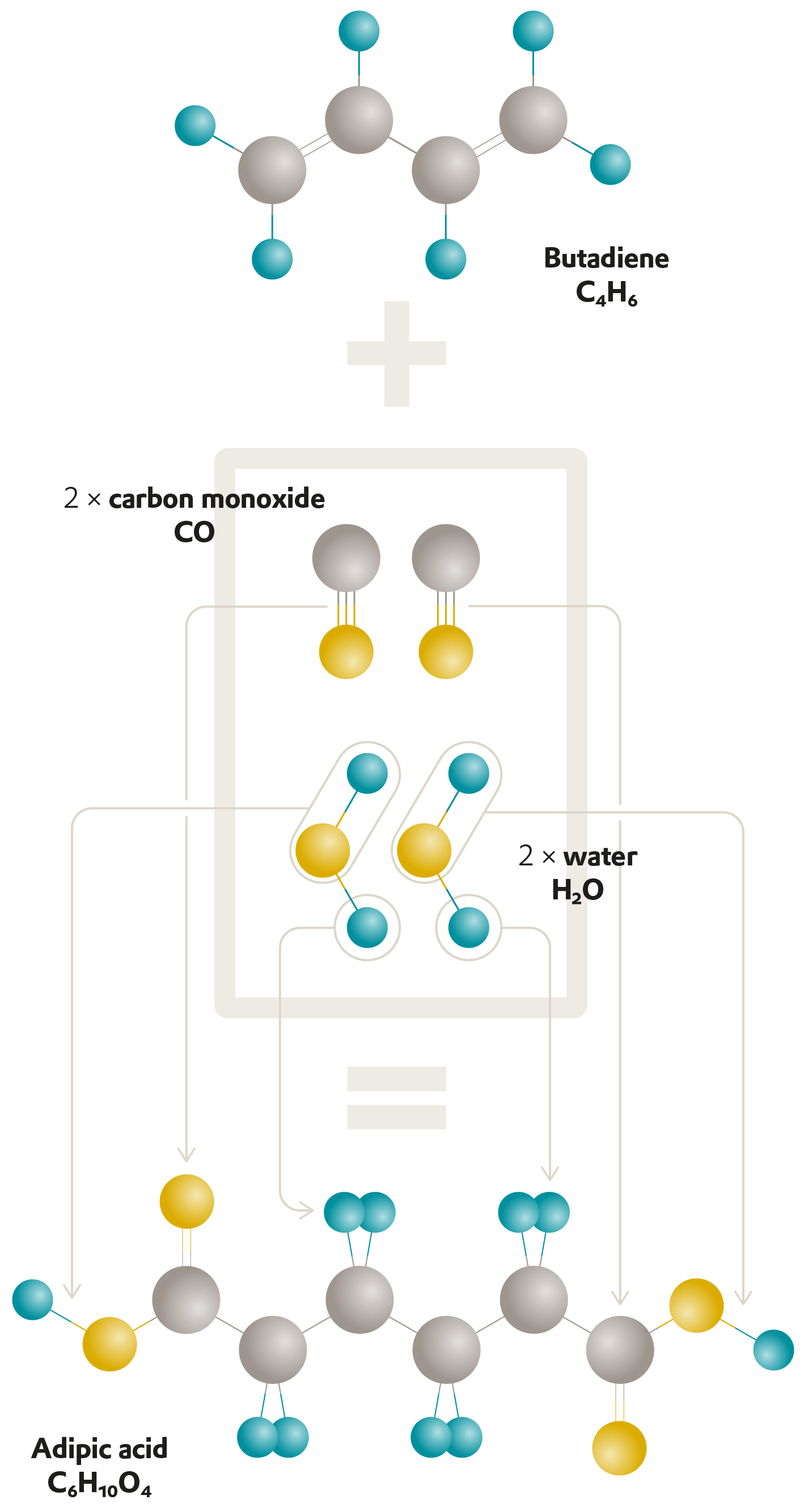
Researchers in the ROMEO project have developed a process that makes an industrial reaction more energy-efficient.

Carbon
The new formula C4
Absolutely unique: Evonik is using an award-winning purification process to make the refinery byproduct FCC-C4 a useful raw material.

Water electrolysis
The membrane is the key
AEM electrolysis is expected to produce large amounts of green hydrogen. A new membrane will make it possible.

Chemical production
The Direct Route
Evonik plans to use a new method to make the production of propylene glycol more sustainable.
ELEMENTS Newsletter
Get fascinating insights into the research Evonik is conducting, and its social relevance, by subscribing to our free newsletter.