Recycling
The plastic cycle
On the path to achieving a true circular economy: Evonik is working on innovative processes in order to reduce plastic waste.

The circular economy
Closing the circle
Lauren Kjeldsen is promoting circularity at Evonik. The ultimate aim is to create a fully functioning circular economy that works without fossil fuels.

The circular economy
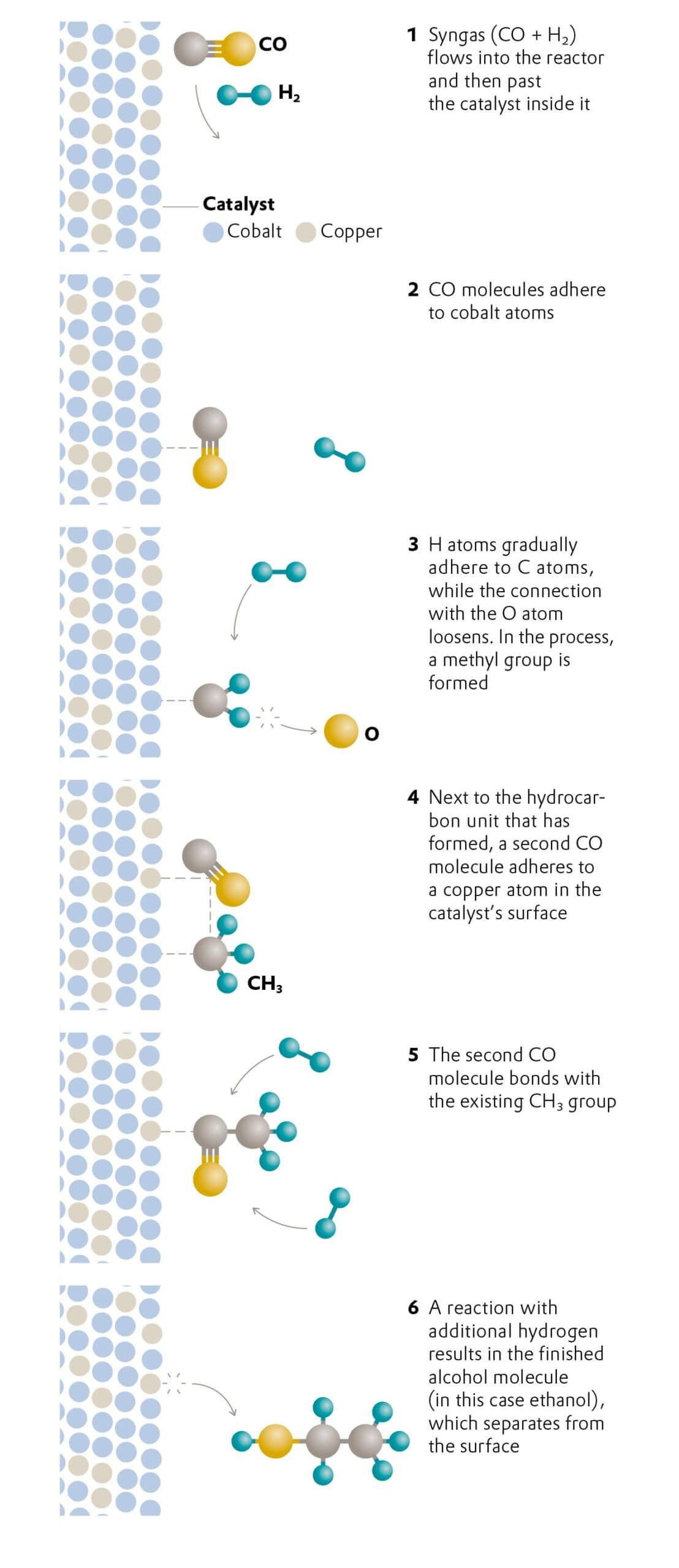
Plastic recycling: Facts and figures
A sustainable cycle: The recycling of plastic requires innovative technologies. An overview.

Plastic
Pathways to the circular economy
Bernhard Bauske and Ingo Sartorius discuss the steps that are needed for the optimal recycling of plastic.
ELEMENTS Newsletter
Get fascinating insights into the research Evonik is conducting, and its social relevance, by subscribing to our free newsletter.