Todd Reith has a list of admirable abilities: software engineer, manufacturing entrepreneur, implant developer, guitar maker. Materials scientist was not one of them when the then Founder and Inventor of FossiLabs in West Chester, Pennsylvania, set out to develop a process to 3D print polyether ether ketone (PEEK) spine implants in 2017.
PEEK is a thermoplastic. The material softens through heating before being processed and left to cool and harden into its desired shape. It’s known for its excellent mechanical and chemical-resistant properties and, therefore, is used in demanding applications across numerous industries, from engine components in cars to fuel systems in aerospace to implants in medtech.
While PEEK was developed more than 40 years ago, it continues to evolve as materials companies produce new formulas and manufacturers build new applications with the polymer. A real quantum leap has now been made by 3D printing the material. It provides patients with spinal disorders better surgical options and thus a better quality of life. This is the result of a fruitful collaboration between a visionary inventor, Reith, and Evonik, a company with an extensive knowledge of materials. Reith’s previous experiences provided him the opportunity to work with different materials and engineer expandable spine cages. That effort led him to believe that 3D-printed PEEK could be a pioneering innovation in orthopedics. He founded FossiLabs, a one-man shop in his basement, and set to work building a 3D printer from scratch. But his limited expertise in the material quickly opened Pandora’s box.
When a patient experiences instability in their spine, a surgeon might remove the intervertebral disc and replace it with an implant. The goal is for the implant to attach or fuse to the vertebra bones above and below the device to reduce motion and stabilize the spine. Thus, implant materials play a critical role in a patient’s recovery process. While every patient responds differently to surgery, implant materials can help with a quicker fusion or decrease the tissue scarring around the implant, both of which are important for the short-term and long-term health of the spine.

PEEK gained adoption in the orthopedic spine market for multiple reasons. The material is radiolucent, allowing physicians to assess the amount of osseointegration, without the implant or artifacts blocking the view in the X-ray or CT image. Furthermore, PEEK nearly matches the modulus of the surrounding bone, making it an ideal material for manufacturers of implants.
However, it cannot attach or integrate the implant to the bone. Over time, companies have launched PEEK spine implants with surface modifications. They injection mold or machine the implant and then add a coating, usually metal, that promotes fixation of the device to the vertebrae. Others began to additively manufacture titanium implants. Additive manufacturing—or 3D printing—allows companies to build structures that let bone grow through and around the device instead of just attaching to its surface.
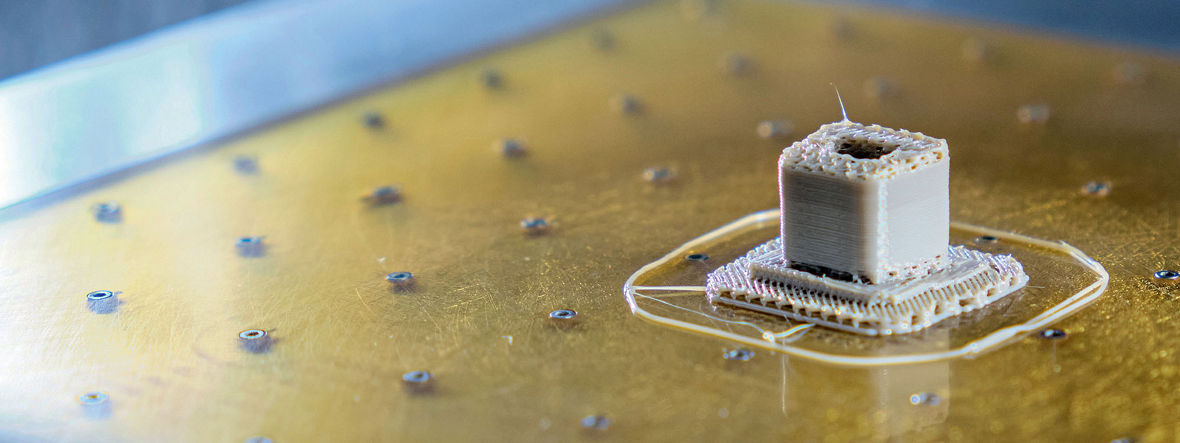
Reith believed that combining the best features of PEEK and 3D printing would create a superior technology. His aim was a fully porous spine implant that mimicked bone and integrated into it. “However, PEEK is nearly impossible to print,” Reith says. “The first challenge was figuring out how to print the material consistently.”
The next big thing
He knew that to achieve his goal, he would need the help of a materials company. Fortunately for him, Evonik’s High Performance Polymers business line was in the development phase of its own implant-grade filament. Reith invited the materials company to see his work in his home lab, and a week later they put their support behind his efforts.
What has transpired over the last five-plus years is a collaboration of a David and a Goliath working together instead of sparing to pioneer innovation in the orthopedic market.
Evonik’s flagship PEEK material, VESTAKEEP®, was launched in 2006 for use in various markets ranging from components in semiconductors to mechanical engineering for food and drinking water applications. Its implantgrade PEEK was first used in a spine device in 2013.

When Evonik saw the early adopters in this area 3D printing PEEK, the company’s medical technology R&D team thought it could revolutionize the field, especially orthopedics—a $59 billion global market in 2023, according to market intelligence firm ORTHOWORLD Inc.
The company also has a rich history of developing materials that can be 3D printed. It, too, believed the marriage of 3D printing and PEEK could respond to surgeon needs and improve patient outcomes. Also, the application could have a wide range of potential, including patient-specific and standard implants for head, face, spine, and trauma applications. There’s even an opportunity for point-of-care manufacturing, where a machine is put in a hospital, allowing surgical care teams to design and print anatomical models and implantable devices as needed. Further, surgeons and medical device companies were requesting implants with porous structures.
“We were visionary enough to say that this could be the next big thing in the market,” says Marc Knebel, Global Segment Head of Medical Devices and Systems and Head of VESTAKEEP® Europe at Evonik.
Believing they could be the leader in this area, Evonik’s global research team got to work to make VESTAKEEP® granules into a 3D-printable filament.

»It was Herculean«
TODD REITH INVENTOR AND VICE PRESIDENT EMERGING TECHNOLOGIES AT CURITEVA
“The good thing is that we are using a material that has been in the implant market for more than ten years with approved applications all over the world,” Knebel says. The technical challenge was to create a filament that had very tight tolerances and fulfilled the requirements for medical implant applications.
Evonik developed a material specifically for fused filament fabrication (FFF), a type of 3D-printing technology that layers the material into a printed part. To achieve this vision, Evonik’s scientists focused on the material’s viscosity, the measure of a fluid’s resistance to flow, and crystallization, the process by which a solid forms.
An advantage of PEEK is that it’s resistant to high temperatures. At the same time, this feature makes it harder to process: Most machines on the market 3D print standard polymers in a temperature range of 200 degrees Celsius, but PEEK needs to reach 400 degrees Celsius to liquefy, Knebel explains. PEEK is also semi-crystalline. Due to crystallization, the material can shrink as the melted structure changes into a hard structure. Evonik’s experts also targeted a tight tolerance, which is especially critical for devices that are implanted in the human body.
The development of VESTAKEEP® i4 3DF was an iterative process that required a balance between the material’s stability and printability. As it was in development, Evonik sent the filament to machine manufacturers worldwide and asked for feedback to further advance the product. This allowed Evonik to identify which manufacturers could produce quality 3D-printed parts. It turned out that Reith was one of them.

Bonded partnership
When Evonik visited his lab in late 2018, Reith had built a proprietary 3D printer. His early request was for Evonik to provide a filament that would satisfy US regulators and help with his understanding of the material chemistry as he perfected his process. They said yes.
Reith and the Evonik team in the United States were in constant communication as he sought to develop a 3D printing process that was highly precise and constantly repeatable. “Todd could make a change the next day if he wanted. We’re slower than him due to our size,” Knebel says. “But our management has given us the freedom to maneuver like a speedboat so that we are able to act and react in a way that startups are expecting.”
The back and forth continued for three years. Reith would print parts, send them to Evonik and ask questions to troubleshoot obstacles, primarily with the crystallinity. Evonik’s Director of Technology, Dr. Suneel Bandi, would review the parts and questions and respond with recommendations for temperature changes.
“I relied more on visual cues, where I was able to discover subtleties in the material properties,” Reith says. When the color was golden honey coming out of the nozzle, he knew he was at the right temperature, viscosity, and flow rate. Then, when the filament would laydown on the build plate, he would watch the speed at which it went to an opaque semi-crystalline state. By controlling the temperature, Reith was ultimately able to control the amorphous state of the material. Evonik’s feedback played a critical role in helping Reith perfect his process, and their collaboration continued when Reith sold FossiLabs to Curiteva, an established spine company based in Huntsville, Alabama.

»We were given
the freedom to
maneuver like a
speedboat«
MARC KNEBEL GLOBAL SEGMENT HEAD OF MEDICAL DEVICES BEI EVONIK
Crucial tests over Christmas
Reith was named Inventor and Vice President of Emerging Technology of Curiteva when the company purchased his firm in 2020. Like Reith, Curiteva was interested in developing novel 3D-printed spine implants and immediately backed his work with the specialty chemicals company. “Evonik and I formed a tight partnership after the acquisition, because then we were on a path to a commercial product,” Reith says. “The acquisition meant we had to turn it into a validated process.”
Reith’s proprietary Fused Strand Deposition (FSD) process prints an implant with diamond-shaped pores with a distribution between 100 and 600 microns (one micron is a thousandth of a millimeter). The design is notable. according to Dr. Erik Erbe, Chief Scientific Officer at Curiteva, because it leads to a stronger implant than can be achieved with compression molded PEEK. “At face value, people might say, ‘Well, that’s just PEEK that they printed differently.’ That’s not the case,” Dr. Erbe says. The company’s studies show that its implant’s compressive strength is 70% to 80% higher than traditional PEEK implants.

Curiteva’s early studies showed that they had developed an implant that could be a potential game-changer for the spine market. Innovation, however, often comes with stringent regulatory hurdles. Evonik launched its VESTAKEEP® i4 3DF implant-grade filament in early 2022. This meant that Curiteva could submit its product for U.S. FDA clearance with a commercial material that met rigorous quality standards. Still, FDA sought affirmation that Curiteva’s printing process didn’t change the characteristics of Evonik’s materials. FDA discussions have strict deadlines, and Curiteva needed to perform intensive chemical analysis in just a few days or risk losing their shot at market clearance.
A journey to Germany
The timing heightened the high-stress moment—it was Christmas. Reith called his contacts at Evonik to phone in a big favor. They said yes again. “Normally at Christmastime, no one is in the office,” Knebel says. “We asked people to stay because we knew it was time-critical.” Reith traveled from the United States to Germany to hand deliver the 3D-printed implants to the Darmstadt facility. Dr. Jonas Scherble, Evonik’s Director of Quality & Regulatory, oversaw the testing. “We have regulatory expertise and supporting data to provide our customers with a good foundation of knowledge,” Knebel says. “It’s all about trust at the end of the day. We want to generate trust with our customers and the authorities by proving that our material is suitable to 3D print implants.” The results of Evonik’s material analysis satisfied the U.S. FDA’s questions, and Curiteva received their clearance. The close collaboration between Curiteva and Evonik resulted in the first 3D-printed PEEK implant to reach the market in the USA—a trailblazing achievement for both companies.
“It was Herculean,” Reith says. “It’s one thing for startups to be quick and agile, but for a company like Evonik to get everyone aligned is impressive. What’s awesome about this story is their willingness to take a risk on a small entrepreneurial company. What’s even more remarkable is that this big company took a risk, and now they’re in a leadership role. All of Evonik’s competitors are playing catch-up now.”
Surgeons performed the first surgery with Curiteva’s Inspire system in April 2023. “I believe structure drives biology and the lattice PEEK architecture enabled by Curiteva’s 3D printing process represents an exciting advancement in spine, orthopedics, and neurosurgical procedures which involve any type of biologic implant,” says Dr. Alex Vaccaro. The president of the Philadelphia-based Rothman Orthopedic Institute is one of the first surgeons using the new implant.

»It’s not just
PEEK that
we’ve printed
differently«
ERIK ERBE CHIEF SCIENTIFIC OFFICER AT CURITEVA

Curiteva sees market potential for various implant applications that can leverage 3D-printed PEEK, including foot and ankle, hand and wrist, and head and face. For example, a patient who endures skull trauma in a car accident could receive a 3D-printed PEEK implant that fits the exact size and shape of their fracture.
Evonik is the only supplier with a 3D-printable implant-grade PEEK material that has been used in an FDA-cleared device. The company is now working with manufacturers across the globe to bring products to market using its VESTAKEEP® i4 3DF materials. “Curiteva’s application created a lot of interest in the market and opened doors for further discussions,” Knebel says. “Not everybody has immediately jumped on using filament. However, it has started discussion and innovation at other companies. That’s a good thing.”
In October 2023, Evonik launched its carbon-fiber-reinforced PEEK filament for 3D-printed medical implants—the next advancement in its materials offerings. The carbon-fiber-reinforced PEEK is expected to be used for spinal cages, trauma plates, and patient-specific applications. The material’s benefit comes from the strength of the high carbon-fiber content matched with the ductility of its PEEK components. It also includes the ability to define the alignment of the carbon fibers during the printing process.
Evonik and Curiteva plan to work together to develop orthopedic implants using the new carbon-fiber PEEK filament. “Now we’re sending Todd back to his lab to start again with the new material,” Knebel says, laughing.