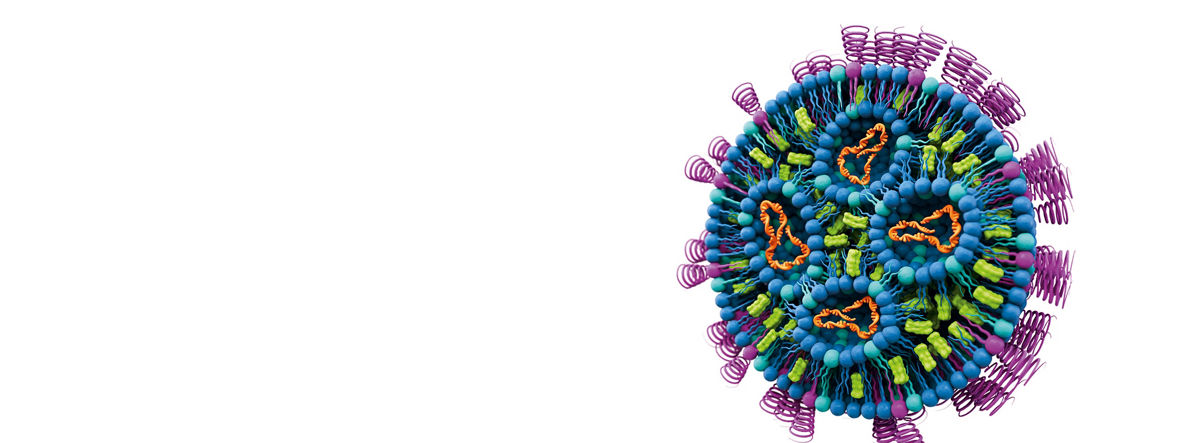
Ms. Engel, many people became aware of lipid nanoparticles during the COVID-19 pandemic. Why are these nanoparticles so important for the field of medicine?
LNPs have been regarded for quite a while now as a great source of hope for medicine, because they are smoothing the path toward medications that are tailored to individual patients. Before the COVID19 pandemic, they were already used to make the active ingredients in cancer medications more tolerable for patients. As a partner of the pharmaceutical industry, we are constantly on the lookout for innovations so that we can continue to improve potential therapies. As the new rPEG lipids are demonstrating, LNPs are an especially innovative area that is likely to develop very fast in the years ahead.
How are you preparing for this boom?
We’re developing new customized lipids, we can formulate active ingredients such as mRNA in LNPs, and we are proficient in the development of the relevant processes. We’ve got very experienced engineering teams that can convert the production of a few milliliters in the laboratory into a stable industrial process that can supply many liters of the product in pharmaceutical quality. Our customers can receive everything from a single source. That accelerates the development of new medications.
Where are your lipid operations based?
For customers in the pharmaceutical industry, it’s important to safeguard the delivery capability of critical starting materials so that vital medications can always be produced. That’s why we’ve distributed the development and production of lipids and LNPs all over the world. In Hanau, lipids are developed and produced in small amounts. At our location in Lafayette, Indiana, in the USA we recently invested in a big plant for the production of lipids for mRNA active ingredients. In Vancouver and Darmstadt we do the early development of formulations for LNPs and conduct the initial tests with them. In Vancouver we also develop the processes for supplying new products for clinical trials.
Who is supporting you in the development process?
We cooperate with several universities. For example, since January 2023 we’ve been a partner in a project supported by the German Federal Ministry for Economic Affairs, in which universities and companies jointly develop new special lipids for mRNA medications. We’re working together with Stanford University in California to create a polymer-based envelope for mRNA. Our aim is to enhance our portfolio of lipid-based products with these polymer particles in the future. And the project in which we cooperate with the University of Mainz focuses on the novel rPEG lipids.