Biotechnology
The green revolution
Undreamed-of opportunities: Biotechnology has a long history and is one of the key sciences of the 21st century.

Innovation
Growth thanks to biotechnology
Is biotechnology a booster for the economy? Which sectors are benefiting from these innovations? An outlook in figures.

Technology assessment
A joint effort in biotechnology
Martina Schraudner, a specialist in molecular biology and scientific theory, is calling for a public debate about biotechnology.

Vaccination
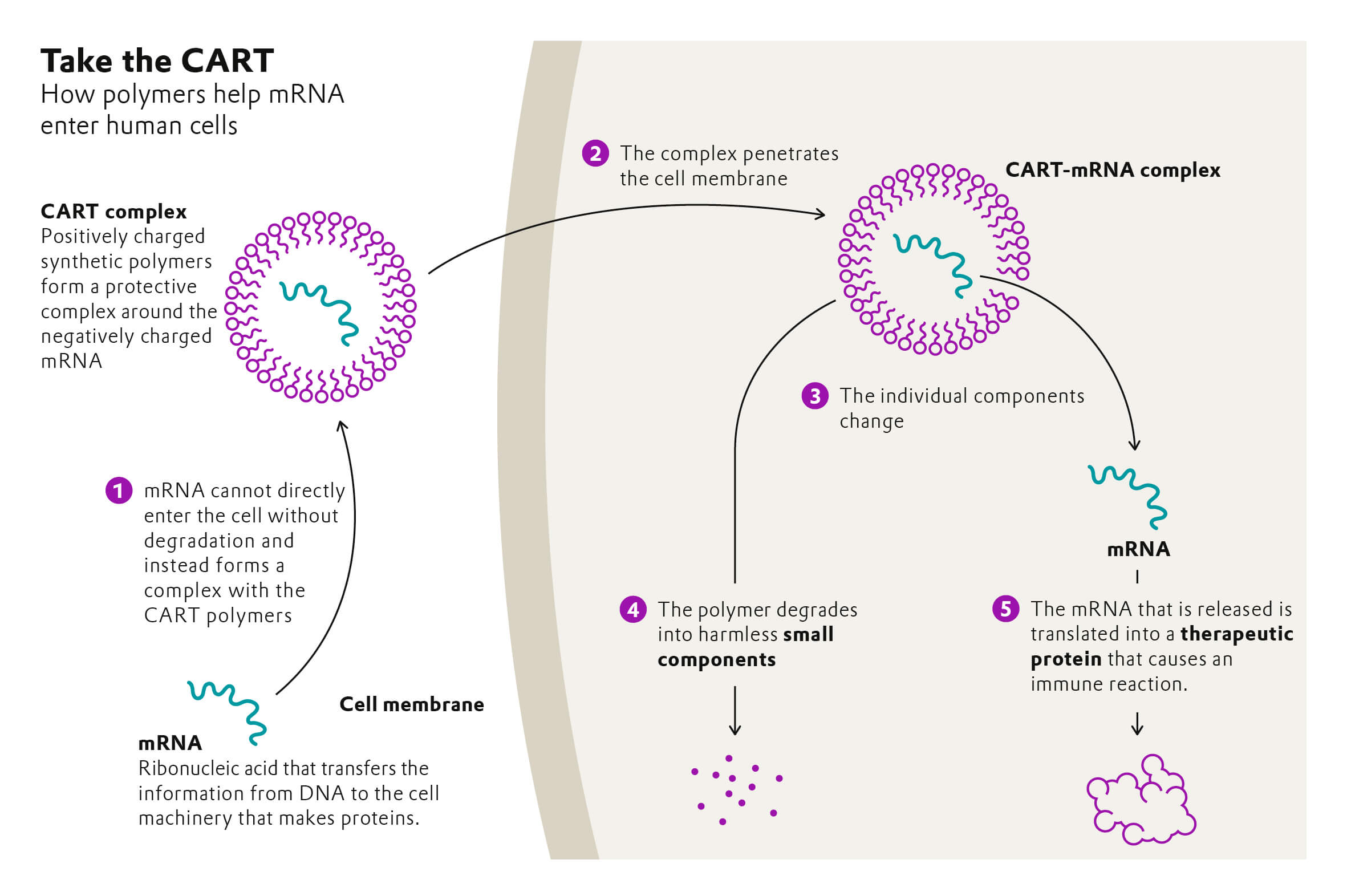
Reliable Transport
Special protection vehicle: Lipid nanoparticles envelop mRNA vaccines and enable their passage through the cell membrane.